We know so little, so little indeed, about solubility
Perlyene diimide (PDI) is famously insoluble, and it’s a well-known belief that linear aliphatic N-substituents won’t do much to improve this.
However, the Grozema group published a mind-blowing work back in 2014 (DOI: 10.1039/c4cc00330f) that completely challenged this common assumption (along with other important findings, of course).
Take a look at the picture: by tweaking the position of the ester group (while maintaining the overall length of the substituents the same), the solubility of PDI increases from negligible to over 100 mg/mL! I’ve known about this work for a while (thanks Pat for showing me this paper), and I still struggle to fully make sense of it. This just goes to show how little we truly understand about intermolecular interactions; there’s so much more to learn!
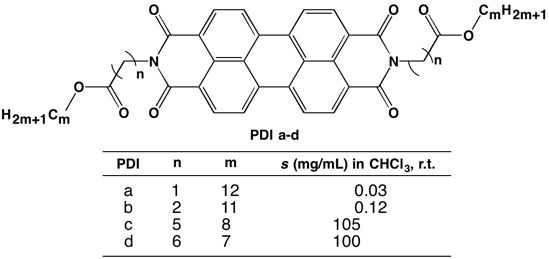
Solubility of PDI derivatives with varying linear N-substituents. (Adapted from DOI: 10.1039/c4cc00330f)